HIDRADENITIS SUPPURATIVA RESEARCH STUDY
RESEARCH STUDY FOR HIDRADENITIS SUPPURATIVA (24 WEEKS)
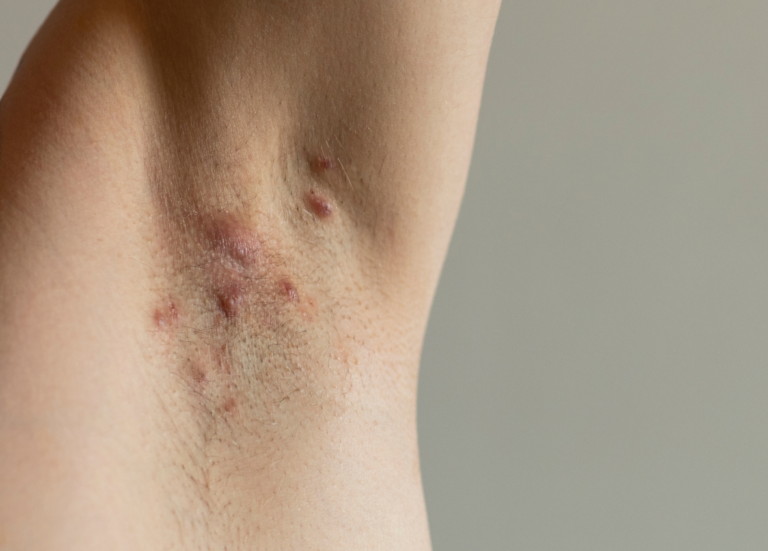
Adults aged 18 years or older who have signs and symptoms of Hidradenitis Suppurativa (HS) for at least 6 months, may qualify for this clinical research trial and receive investigational injected treatment
(active study drug) or placebo.
Eligible participants must have moderate to severe HS defined as: a total of at least 5 inflammatory lesions, and HS inflammatory lesions present in at least 2 distinct anatomic areas. Participants will have regular visits with an experienced research team. There is no cost to participate, and participants will be reimbursed for study-related expenses.
Individuals will be asked to visit the study centre 12 times over a period of about 24 weeks.
For further information on the study, please follow the link:
Principal Investigator
A/Prof Peter Foley
Sub Investigators
Dr Conor Larney
t: 9623 9464
e: [email protected]
Dr Laxmi Iyengar
t: 9623 9464
e: [email protected]
Study Coordinator
Asami Weaver
t: 9623 9439
e: [email protected]
