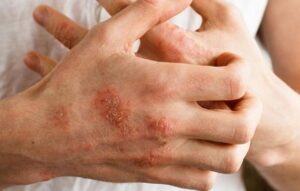
RESEARCH TRIAL FOR ATOPIC HAND AND FOOT DERMATITIS (32 WEEKS)
Adults aged 18 years or older who have hand and/or foot eczema (atopic dermatitis), may qualify for this clinical research trial and receive investigational injected treatment (active study drug) or placebo. Eligible participants must have a diagnosis of chronic atopic hand and/or foot dermatitis for at least 1
year. Atopic dermatitis (AD) must be present in at least 2 of the 4 mentioned anatomical areas: left hand, right hand, left foot, or right foot. Participants must have overall moderate-to-severe disease in the hands/feet, along with a documented history of inadequate response to topical corticosteroids (TCS) within 6 months, or use of TCS is medically inadvisable.
Participants will have regular visits with an experienced research team. There is no cost to participate, and participants will be reimbursed for study-related expenses. Individuals will be asked to visit the study centre about 8 times over a period of about 32 weeks.
For more detailed information about this clinical trial, please visit:
Principal Investigator
A/Prof Peter Foley
Sub Investigators
Dr Conor Larney
Phone Number: (03) 9623 9464
Email: [email protected]
Dr Laxmi Iyengar
Phone Number: (03) 9623 9464 Email: [email protected]
Study Coordinator
Tracy Mallett
Phone Number: 03 9623 9439
Email: [email protected]
COMPARATOR TRIAL FOR MODERATE TO SEVERE ECZEMA
Adults aged 18 years or older who have a diagnosis of eczema / atopic dermatitis (AD) that has been present for 1 year or longer, may qualify for this clinical research trial and receive investigational injected treatment (active study drug) or comparator medication.
Eligible participants must have moderate to severe eczema / atopic dermatitis (AD) affecting 10% or more of their body surface area (this equates to 10 palm prints). A history of poor response to treatment with topical medications, or medical determination that topical therapies are inadvisable, is also required. A non-medicated over-the-counter emollient/moisturizer must be used throughout the study.
Participants will have regular visits with an experienced research team. There is no cost to participate, and participants will be reimbursed for study-related expenses. Participants will be asked to visit the study centre about 24 times over a period of about 82 weeks.
Principal Investigator
A/Prof Peter Foley
Sub Investigators
Dr Conor Larney
Phone Number: (03) 9623 9464
Email: [email protected]
Dr Laxmi Iyengar
Phone Number: (03) 9623 9464 Email: [email protected]
Study Coordinator
Tracy Mallett
Phone Number: 03 9623 9439
Email: [email protected]